Back
At its 2025 annual conference, the International Society for Cell and Gene Therapy (ISCT) decisively released an updated set of identification criteria for Mesenchymal Stromal Cells (MSCs) [1]. Unlike the previous 2006 standards [2], the new version explicitly defines MSCs as "mesenchymal stromal cells" rather than "stem cells." Just before the release of these new criteria, in late 2024, Mesoblast’s Ryoncil®—previously marketed as a "stem cell" therapy—achieved a landmark milestone by gaining FDA approval as the first MSC-based drug, rebranded under its scientifically accurate identity as "mesenchymal stromal cells." With a staggering price tag of $1.55 million per treatment (approximately over 11 million RMB), the drug sparked widespread debate. This raises critical questions: What exactly are MSCs? Why has their controversial identity not deterred intense interest? What functions justify their exorbitant cost, and how can they be used safely and effectively? Behind these questions lies not just the astonishing financial figures driven by MSC reclassification, but also a three-decade-long cognitive revolution in mesenchymal stromal cell therapy.
1. Historical Quandary: The Scientific Ambiguity Under the "Stem Cell" Label
In 1991, Professor Arnold Caplan from Case Western Reserve University in the United States introduced the term "mesenchymal stem cells (MSCs)" [3] based on his scientific research and preclinical trials, describing a unique cell population found in mesenchymal tissues that possessed proliferative and in vitro differentiation capabilities. These cells generated significant enthusiasm due to their remarkable potential in tissue regeneration and repair [3-4].
In 1992, following the discovery of MSCs, Osiris Pharmaceuticals was established to develop MSC-based therapeutics.
In 2012, the U.S. FDA rejected Osiris's marketing application for its MSC drug Prochymal®. The FDA determined there was a disconnect between its claimed stem cell repair mechanism and actual clinical data, along with a lack of validated potency assay data to support efficacy. This conclusion was documented in Osiris's 2012 SEC filings and referenced in the FDA's 2017 Cell Therapy Guidance, which stated: "Prochymal® lacks validated potency assays, a clearly defined mechanism of action, and definitive evidence demonstrating that MSCs function as stem cells with differentiation capacity in vivo."
In 2013, Mesoblast acquired Prochymal® and rebranded it as Ryoncil® (remestemcel-L). However, the retention of the "-stem-" prefix in its nonproprietary name indicated the development team still regarded it as a stem cell product, foreshadowing the challenging regulatory path ahead.
In May 2019, Ryoncil® submitted its first Biologics License Application (BLA) to the FDA, which was rejected primarily due to insufficient single-arm trial evidence and inadequate Chemistry, Manufacturing, and Controls (CMC) data.
In August 2023, Ryoncil® resubmitted its BLA. Despite including four years of subject survival data, the application was again rejected by the FDA due to the absence of adult patient control studies and persistent CMC deficiencies.
These repeated regulatory setbacks exposed critical flaws in positioning MSCs as stem cell therapeutics. Although labeled as mesenchymal stem cells, Ryoncil®'s clinical trials failed to demonstrate that tissue differentiation mediated its reparative effects. Conversely, substantial clinical evidence indicates that MSCs exert their therapeutic benefits primarily through microenvironment modulation following tissue homing - a mechanism fundamentally distinct from classical stem cell activity.
2. Academic Exploration: The Quest for Truth - From "Mesenchymal Stem Cells" Back to "Mesenchymal Stromal Cells"
In 2016, Professor Caplan, hailed as the "father of mesenchymal stem cells," corrected his 25-year-old definition of MSCs through experimental research and clinical observations. He redefined MSCs as "mesenchymal stromal cells" or "medicinal signaling cells" [5]. This redefinition proved crucial for MSC therapeutic applications. However, due to the absence of clear identification standards to definitively distinguish MSCs from stem cells, their identity remained controversial for an extended period, with most cases still erroneously labeled as stem cells.
In August 2022, the stem cell research team at Tasly Pharmaceutical published a groundbreaking paper titled "Transcriptomic heterogeneity of cultured ADSCs corresponds to embolic risk in the host" in iSCIENCE [6]. This study pioneered the use of single-cell RNA sequencing combined with AI modeling to systematically analyze MSC transcriptomic characteristics at single-cell resolution. Their results conclusively demonstrated that the examined cells were mesenchymal stromal cells, not stem cells.
In February 2025, Dr. Yan Kaijing from Tasly and colleagues published another seminal paper in Heliyon titled "Unveiling distinctions between mesenchymal stromal cells and stem cells by single-cell transcriptomic analysis" [7]. This landmark study represented the world's first definitive elucidation of fundamental differences between MSCs and stem cells. Through single-cell RNA sequencing and pseudotime trajectory analysis, the research team identified specific biomarkers to distinguish MSCs from stem cells, laying the core foundation for updating MSC standards.
In May 2025, ISCT officially released new MSC identification criteria at its annual conference, explicitly defining MSCs as mesenchymal stromal cells. The updated standards mandate that any use of the term "stem cells" (i.e., mesenchymal stem cells) must be supported by experimental evidence of stemness. Previous stemness evaluation criteria (in vitro trilineage differentiation potential) and standard adherent culture conditions were excluded from the new guidelines. The revised standards also refined cell marker requirements, established flow cytometry thresholds and percentage criteria, emphasized tissue source documentation, and strengthened critical quality attribute assessments for potency and characteristics. These updates finally resolved the longstanding identity debate in the field, paving the way for returning to authentic mesenchymal stromal cell therapy [1].
The following timeline (Figure 1) summarizes the cognitive revolution in mesenchymal stromal cell therapy from 1991 to present:
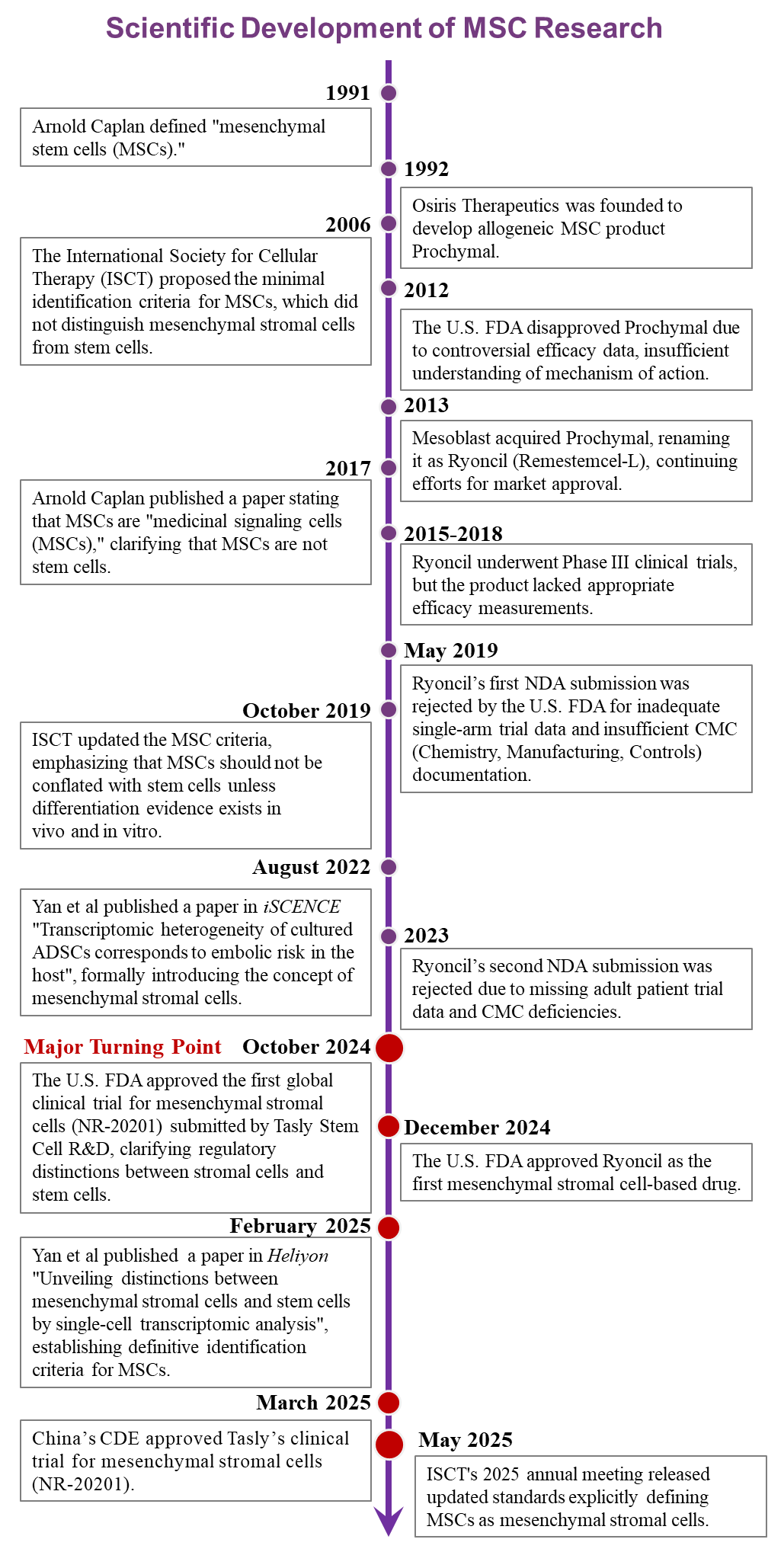
Figure 1. Evolution of the Conceptual Revolution in Mesenchymal Stromal Cell Therapy
3. Regulatory Advancements: MSC Industry Reaches Historic Breakthrough
As scientific understanding has deepened, regulatory agencies have rapidly updated their perspectives on mesenchymal stromal cells (MSCs) versus mesenchymal stem cells. In October 2024, the U.S. FDA approved the world’s first MSC-based therapy with the explicit designation "mesenchymal stromal cells" in its Investigational New Drug (IND) application, submitted by Tasly Pharmaceutical’s research team [8]. Later that year, the FDA further granted approval to Ryoncil®, the first MSC drug to be officially classified as a "mesenchymal stromal cell therapy" [9]. These decisions marked a paradigm shift in regulatory recognition of MSCs.
In March 2025, China’s Center for Drug Evaluation (CDE) under the National Medical Products Administration (NMPA) approved Tasly’s IND application for "Allogeneic Adipose-Derived Mesenchymal Stromal Cell Injection", ushering in a new era of MSC-based clinical trials in China [10].
4. Conclusion
Since their discovery, mesenchymal stromal cells (MSCs) have captivated global attention due to their unparalleled therapeutic potential for a wide range of serious diseases. Despite the proliferation of MSC clinical trials, therapeutic efficacy has remained inconsistent, with only a handful of products successfully gaining regulatory approval. "Flowers without fruit"—this phrase has come to symbolize the persistent frustration in the MSC industry. The root cause lies in the fundamental difference between MSC mechanisms and those of stem cells: MSCs must effectively home to injured tissues, interact with the microenvironment, and release signaling factors to exert their reparative effects. Thus, the timeliness and efficiency of homing determine therapeutic outcomes. Yet, from their discovery in 1991 through three decades of clinical exploration, MSCs were erroneously classified as stem cells, leading to misguided therapeutic approaches. This misalignment not only prevented MSCs from fulfilling stem cell-like functions but also hindered their innate biological potential, resulting in unreliable efficacy. "If the name is not correct, the justification will not stand"—regulatory agencies naturally could not approve products with unclear mechanisms and unpredictable effects. The tide began to turn in late 2024, when the FDA approved the first MSC clinical trial explicitly designated as "mesenchymal stromal cell therapy." This milestone marked the formal recognition of MSCs' true biological identity and initiated the correction of decades of conceptual confusion.
For the public, what matters is not what MSCs are called, but whether they are safe and effective. Unlike stem cells, MSCs lack tumorigenic risks, making them inherently safer. As for efficacy, the recent authoritative redefinition of MSCs by international organizations is pivotal—it establishes a mechanism-driven therapeutic framework to ensure reliability. The updated standards signify that MSC therapies have entered a new era, one that will inevitably trigger a global revolution across academia, regulatory bodies, and industry.
Looking back on MSC’s 30-year journey—marked by scientific debates, regulatory challenges, and relentless pursuit of medical breakthroughs—we now stand at a turning point. Cell therapy is transitioning from the vague concept of "mesenchymal stem cell infusion" to precise "personalized mesenchymal stromal cell medicine." The healthcare paradigm is shifting from "disease-centric" to "patient-centric" care. Who will seize this transformative opportunity to truly advance MSC therapies from bench to bedside? The future of regenerative medicine is here, and its impact on human health awaits.
[1] Renesme L, Cobey K D, Lalu M M, et al. Delphi-driven consensus definition for mesenchymal stromal cells and clinical reporting guidelines for mesenchymal stromal cell–based therapeutics[J]. Cytotherapy, 2025, 27(2): 146-168.
[2] M. Dominici, MD et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; Volume 8:315-317.
[3] Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991 Sep;9(5):641-50.
[4] Caplan AI. Bone development and repair[J]. Bioessays, 1987, 6(4): 171-175.
[5] Caplan AI. MSCs: The Sentinel and Safe-Guards of Injury. J Cell Physiol. 2016 Jul;231(7):1413-6.
[6] Yan K, Zhang J, Yin W, Harding JN, Ma F, Wu D, Deng H, Han P, Li R, Peng H, Song X, Kang YJ. Transcriptomic heterogeneity of cultured ADSCs corresponds to embolic risk in the host. iScience. 2022 Aug 4;25(8):104822.
[7] Yan K, Ma F, Song X, Wang H, Liu P, Zhang J, Jin X, Han P, Zuo X, Kang YJ. Unveiling distinctions between mesenchymal stromal cells and stem cells by single-cell transcriptomic analysis. Heliyon. 2025 Feb 8;11(4):e42311.
[8] FDA Investigational New Drug Application: NO.30788
[9] FDA Approves First Mesenchymal Stromal Cell Therapy Treat Steroid Refractory Acute Graft Versus Host
[10]NMPA CDE Acceptance Number:CXSL2400882